Scattering Of Light

Blue Sky
The blue color of the sky is caused by the scattering of sunlight off the molecules of the atmosphere. This scattering, called Rayleigh scattering, is more effective at short wavelengths (the blue end of the visible spectrum). Therefore the light scattered down to the earth at a large angle with respect to the direction of the sun's light is predominantly in the blue end of the spectrum.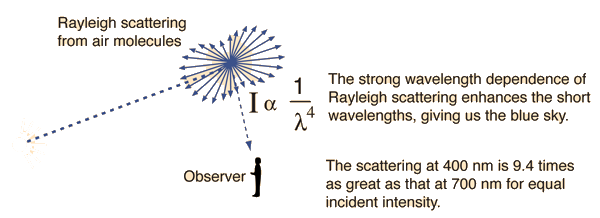
 | Note that the blue of the sky is more saturated when you look further from the sun. The almost white scattering near the sun can be attributed to Mie scattering, which is not very wavelength dependent. |
| Clouds in contrast to the blue sky appear white to achromatic gray. |  |
Rayleigh Scattering
Rayleigh scattering refers to the scattering of light off of the molecules of the air, and can be extended to scattering from particles up to about a tenth of the wavelength of the light. It is Rayleigh scattering off the molecules of the air which gives us the blue sky. Lord Rayleigh calculated the scattered intensity from dipole scatters much smaller than the wavelength to be: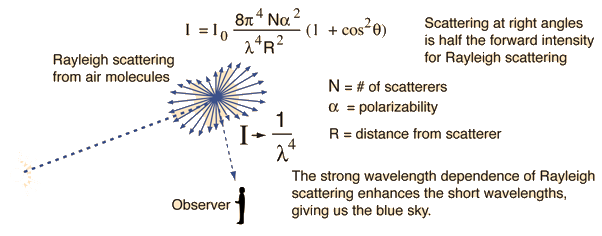
Mie Scattering
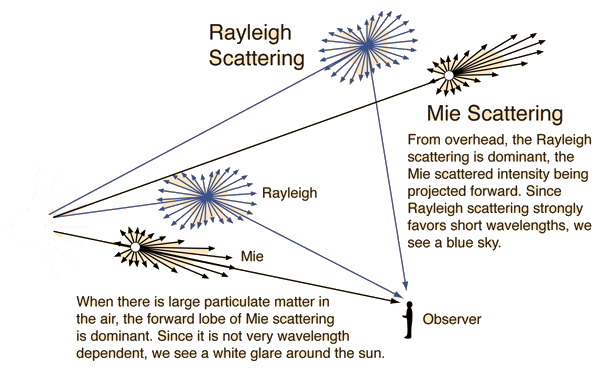
The scattering from molecules and very tiny particles (< 1 /10 wavelength) is predominantly Rayleigh scattering. For particle sizes larger than a wavelength, Mie scattering predominates. This scattering produces a pattern like an antenna lobe, with a sharper and more intense forward lobe for larger particles.
Greenler in his "Rainbows, Haloes and Glories" has some excellent color plates demonstrating Mie scattering and its dramatic absence in the particle-free air of the polar regions.
Comparing Rayleigh Scattering with Mie Scattering
Raman Scattering
Like Rayleigh scattering, the Raman scattering depends upon the polarizability of the molecules. For polarizable molecules, the incident photon energy can excite vibrational modes of the molecules, yielding scattered photons which are diminished in energy by the amount of the vibrational transition energies. A spectral analysis of the scattered light under these circumstances will reveal spectral satellite lines below the Rayleigh scattering peak at the incident frequency. Such lines are called "Stokes lines". If there is significant excitation of vibrational excited states of the scattering molecules, then it is also possible to observe scattering at frequencies above the incident frequency as the vibrational energy is added to the incident photon energy. These lines, generally weaker, are called anti-Stokes lines.
Although finding some application in vibrational spectroscopy of molecules, the use of direct infrared sources for such spectroscopy is usually much easier. Raman spectroscopy has found some application in remote monitoring for pollutants. For example, the scattering produced by a laser beam directed on the plume from an industrial smokestack can be used to monitor the effluent for levels of molecules which will produce recognizable Raman lines.
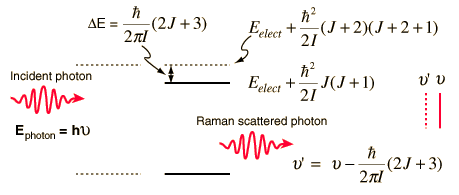
Raman scattering can also involve rotational transitions of the molecules from which the scattering occurs. Thornton and Rex picture a photon of energy slightly than the energy separation of two levels being scattered, with the excess energy released in the form of a photon of lower energy. Since this is a two-photon process, the selection rule is DJ = +/-2 for rotational Raman transitions. The sketch below is an idealized depiction of a Raman line produced by interaction of a photon with a diatomic molecule for which the rotational energy levels depend upon one moment of inertia. The upper electronic state of such a molecule can have different levels of rotational and vibrational energy. In this case the upper state is shown as being in rotational state J with scattering associated with an incoming photon at energy matching the J+2 state.
In Raman scattering, an intense monochromatic light source (laser) can give scattered light which includes one or more "side-bands" that are offset by rotational and/or vibrational energy differences. This is potentially very useful for remote sensing, since the side-band frequencies contain information about the scattering medium which could be useful for identification. Current projects envision Raman scattering as a tool for identification of mineral forms on Mars. Such remote sensing could become a major tool in planetary exploration.
Raman Scattering On Minerals
Raman scattering can be used as a tool for the identification of minerals. Since the Raman spectra for different mineral tend to have sharp peaks which form a fairly unique pattern, they can serve as "fingerprints" for minerals. Since the Raman spectra can be collected remotely, they show great promise for planetary exploration.
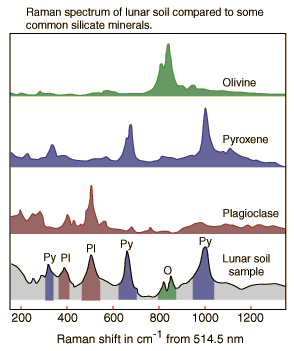 | The illustration at left shows qualitative sketches of Raman spectra displayed by the Department of Earth and Planetary Sciences, Washington University in St. Louis. This research group is developing Raman spectrometers for in situ analysis of minerals on planetary surfaces. The spectra are attributed to Wang et al., J. Geophys. Res. 100, p21189-21199 (1995). Spectra of the common silicate minerals olivine, pyroxene, and plagioclase are compared to a Raman spectrum of a lunar soil sample identified as 71501. |
Watch Video: